![[Photo of woman's hand signing informed consent document]](https://prenatalinformation.org/wp-content/uploads/2015/03/hand-signing-form-closeup.jpg?w=300&h=244) The PRenatal INformed Consent Evaluation & Synthesis Study (PRINCESS) is evaluated over 30 informed consent documents for non-invasive prenatal genetic screening. These documents were collected from 11 countries and a variety of sources, including individual clinics and large commercial test developers.
The PRenatal INformed Consent Evaluation & Synthesis Study (PRINCESS) is evaluated over 30 informed consent documents for non-invasive prenatal genetic screening. These documents were collected from 11 countries and a variety of sources, including individual clinics and large commercial test developers.
Papers from this study:
Marsha Michie, Stephanie A. Kraft, Mollie A. Minear, Roberta R. Ryan, and Megan A. Allyse. (2016) “I have read and I understand”: Informed consent documentation for noninvasive prenatal genetic screening. Journal of Medicine, Ethics, and Public Health.
Presentations from this study:
In March 2016, we presented the completed results at American College of Medical Genetics and Genomics (ACMG) in Tampa, FL. 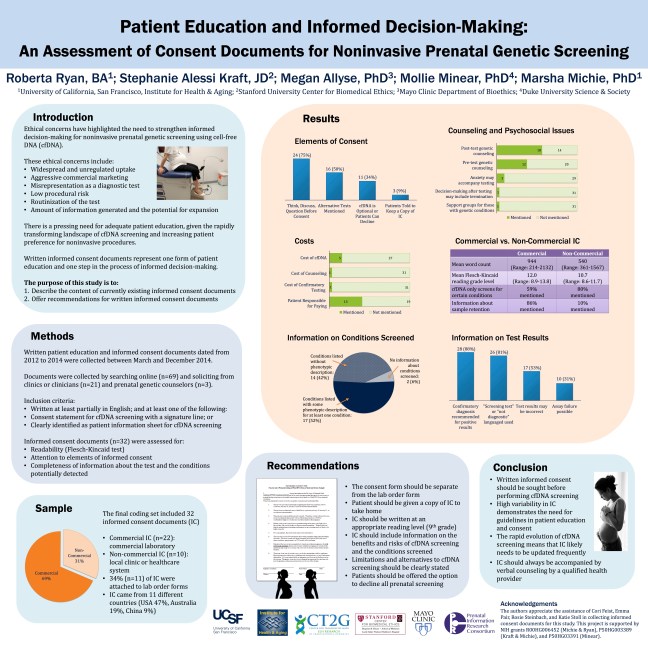
We presented preliminary results from this study at the American College of Medical Genetics and Genomics (ACMG) meeting in Salt Lake City, Utah, in March, 2015.
![[Click to open full size]](https://prenatalinformation.org/wp-content/uploads/2015/03/acmg-2015-princess-thumbnail.jpg?w=648) If you landed here after viewing our poster at ACMG 2015 and would like to take a closer look online, click the thumbnail at left to view a larger image. For more information, to give us feedback on this project, or to request notifications about the eventual publication, please contact us.
If you landed here after viewing our poster at ACMG 2015 and would like to take a closer look online, click the thumbnail at left to view a larger image. For more information, to give us feedback on this project, or to request notifications about the eventual publication, please contact us.
We welcome your feedback and hope to hear from you!

4 thoughts on “PRenatal INformed Consent Evaluation & Synthesis Study (PRINCESS)”