In 2016 the American College of Medical Genetics and Genomics (ACMG) issued guidelines for laboratories offering non-invasive prenatal screening (NIPS). In 2018 a team of collaborators evaluated laboratories currently offering NIPS based on these guidelines. The original paper reporting these results has been published in Genetics in Medicine.
Our aim is to keep those evaluations up to date in table format here. We will revisit our evaluations regularly; however, if you believe any portion of this table should be updated, please Contact Us.
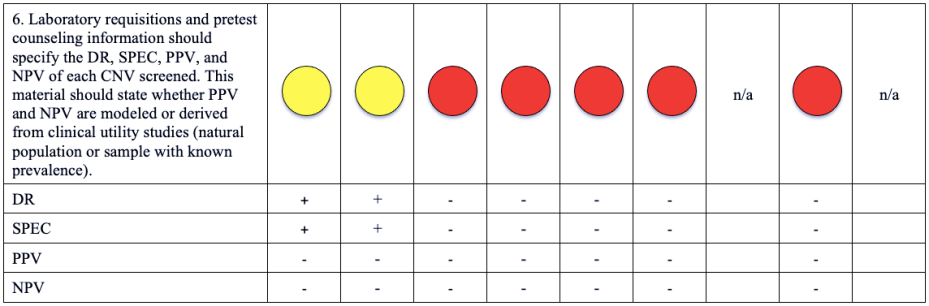
Please note that a green dot indicates “full adherence” to the 2016 ACMG guidelines; a yellow dot indicates “partial adherence,” and a red dot indicates “little to no evidence of adherence.” In some cases we had insufficient information to assess adherence and marked those categories as “could not assess.”
Table: Adherence of Cell-free DNA Noninvasive Prenatal Screening Tests to Laboratory Recommendation in ACMG Position Statement
Table last updated: June 14, 2021
For more information about this study, please see the full publication:
Skotko BG, Allyse M, Bajaj K, Best BG, Klugman S, Leach M, Meredith S, Michie M, Stoll K, Gregg AR. Adherence of Cell-free DNA Noninvasive Prenatal Screens to ACMG Recommendations. Genetics in Medicine 2019 Oct;21(10):2285-2292.